Whats the go for steel or copper wool for scrubbing rust (i don't believe any pitting). Ive got a 1500 Howa stainless steel and 2 greasy paws that didn't oil the barrel (lesson learned). Wondering on size and where to get it? cheers.
Welcome guest, is this your first visit? Create Account now to join.
Welcome to the NZ Hunting and Shooting Forums.
Search Forums
User Tag List
+ Reply to Thread
Results 1 to 15 of 25
Thread: Steel/Copper wool for rust
-
27-05-2020, 02:15 PM #1Member

- Join Date
- Apr 2020
- Location
- Christchurch
- Posts
- 9
Steel/Copper wool for rust
-
-
27-05-2020, 02:26 PM #2Member

- Join Date
- Jun 2015
- Location
- christchurch
- Posts
- 18,676
decorating aisle at miter 10, i got "sifa" brand and it comes in varying wire thicknesses. i used it to matt down an oil finished wood stock
-
27-05-2020, 02:42 PM #3
Copper on stain less would be worse,any fragments left behind will form a chemical cell which causes electrolysis.
-
27-05-2020, 03:33 PM #4Member

- Join Date
- Mar 2014
- Location
- Tauranga
- Posts
- 3,044
-
27-05-2020, 05:15 PM #5
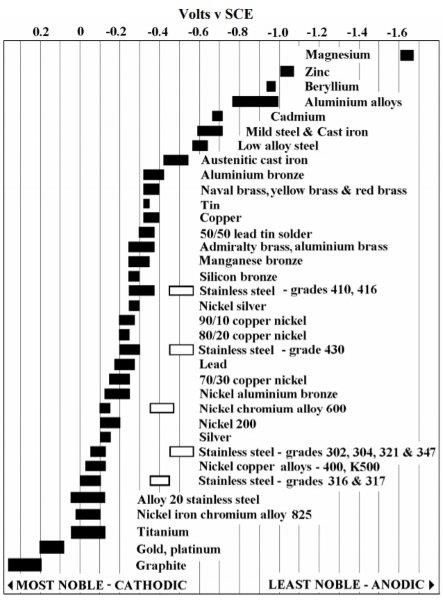
No not really... copper on aluminium is a problem, especially in the presence of an electrolyte... sea water, lemon juice, salts, bleaches and perspiration etc
Copper on stainless will cause the copper to corrode not the stainless, but only just and over a long time.... there are some copper alloys, bronzes or copper nickel alloys that could cause corrosion marks on stainless in time.... and also as some one pointed out ... there is a huge range of stainless grades, some less noble than others.
On the chart above, the further apart the metals (Left and right) the more likely you will have galvanic corrosion if they are in contact... and a metal to the right (less noble) will always sacrifice to a metal to the left. (more noble)
For a nice matt finish on stainless... I just use green scotch brite pads,... bit of CRC.... I am not sure what scotch brite will do to blueing.
An interesting aside for the Nautical minded, Galvanized steel bolts cause less corrosion to Aluminium than a stainless bolt will (There are compounds to help... no not copper coat) .... and this principal is the reason you never leave lead sinkers in the bilge of your tinny.
-
27-05-2020, 07:08 PM #6Member

- Join Date
- Jan 2018
- Location
- kaiapoi
- Posts
- 7,484
CRC is a shit gun oil anyway. WD40 would be the minimum of the mainstream industrial spray can lubricants.
-
27-05-2020, 07:46 PM #7Member

- Join Date
- Dec 2011
- Location
- NI
- Posts
- 13,610
Ive always just used a bit of kitchen Jiff on stainless. Rub it with my fingers or a soft cloth.
-
27-05-2020, 07:53 PM #8Member

- Join Date
- Jan 2018
- Location
- kaiapoi
- Posts
- 7,484
-
27-05-2020, 08:17 PM #9Member

- Join Date
- Feb 2014
- Location
- Hawkes Bay
- Posts
- 2,734
Not necessarily:
Anti-Corrosion Products Test — Video Reveals Best Rust Blockers « Daily Bulletin
Personally I rate Corrosion X. Not even a hint of rusting on any of my toys after I shifted to using it
-
27-05-2020, 09:24 PM #10Member

- Join Date
- Aug 2019
- Location
- South Otago
- Posts
- 4,018
-
28-05-2020, 12:32 AM #11Member

- Join Date
- Apr 2020
- Location
- Christchurch
- Posts
- 9
Yea I'm using whatever gun oil came with my cleaning kit. I went out before lockdown and then didn't oil barrel when I stored it
-
28-05-2020, 02:17 AM #12Member

- Join Date
- Jan 2017
- Location
- Waiuku
- Posts
- 852
-
28-05-2020, 09:10 AM #13Member

- Join Date
- Mar 2014
- Location
- Tauranga
- Posts
- 3,044
-
01-06-2020, 08:15 AM #14Member

- Join Date
- Jan 2018
- Location
- Helensville, Auckland
- Posts
- 473
Wet screwed up tin foil takes it straight off did it on a friends howa ,only stainless though would make a mess of blueing.
-
01-06-2020, 09:55 AM #15
none of the above...
The link below shows you how to boil your gun.
Looks like the business, this guy has some really informative videos.
https://www.youtube.com/watch?v=arcosYVsfCM&t=1334s
He has a video somewhere which goes into detail about the "carding wheel" he uses to polish the scum off of the blued steel after boiling.
I cant find that video, I suspect he has restricted the video to his Patreon subscribers, I think Ill sign up as his videos are bloody good.Use enough gun
Similar Threads
-
KG 12 copper remover
By wsm junkie in forum Reloading and BallisticsReplies: 37Last Post: 23-05-2018, 09:18 AM -
The many uses of the Cotton Wool Bud
By WillB in forum Firearms, Optics and AccessoriesReplies: 0Last Post: 26-10-2016, 08:03 AM
Tags for this Thread
Welcome to NZ Hunting and Shooting Forums! We see you're new here, or arn't logged in. Create an account, and Login for full access including our FREE BUY and SELL section Register NOW!!




 13Likes
13Likes LinkBack URL
LinkBack URL About LinkBacks
About LinkBacks


 Reply With Quote
Reply With Quote


Bookmarks